Abstract
Introduction:
Cardiovascular disease is the leading cause of death, in Uruguay it corresponds to 30%. To the known risk factors, the use of QT and RT are added. The improvement in response and overall survival of hematologic patients allow a longer time to develop cardiovascular complications. Cardiotoxicity has been extensively studied in the context of breast cancer and the use of anthracyclines; however, there are very few data on hematopoietic stem cell transplantation (HSCT).
Objective:
Assess subclinical myocardial damage by measuring biomarkers and echocardiography and identify patients at high risk of developing cardiotoxicity after HSCT.
Methods:
This is a prospective, single-center study between April 2017 and November 2020. Population: adult patients admitted in the British Hospital Transplant Unit, Montevideo Uruguay to receive either an autologous or allogeneic HSCT. Measurement: cardiac biomarkers (pro-BNP, Troponin T, Troponin I and CPK) at admission, D1, D14 and D30. Echocardiograms were performed on admission and at D30 by the same team of 3 echocardiographers with the same machine. It was repeated at D100 if some alteration was seen.
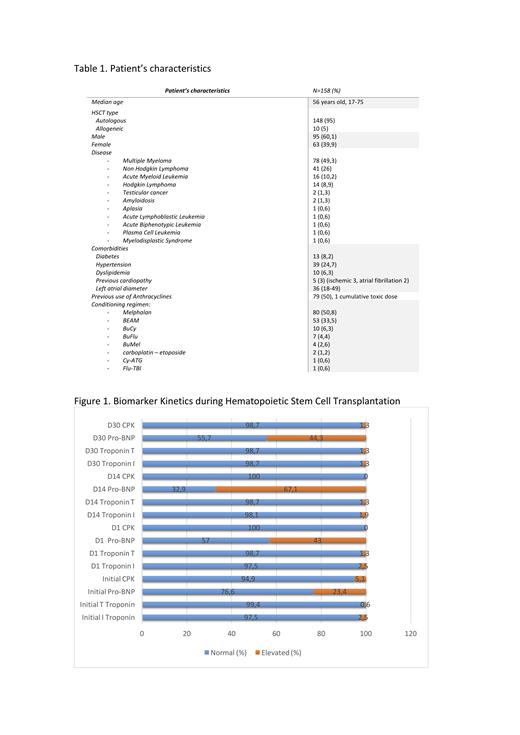
Results:
We included 158 transplants: 148 autologous and 10 allogeneic. The characteristics of the population and results are shown in Table 1. 126 raised some biomarker during the first 100 days (79.7%). Pro-BNP is the biomarker that most frequently rises after admission until day 100: 125/158 (79.1%). The kinetics of the biomarkers are shown in Figure 1. Regarding echocardiograms, there were no patients with a cardiotoxicity criterion defined by: a decrease in LVEF of more than 10% to a value less than 53%. Regarding myocardial deformability, there was a reduction in strain between the initial echocardiogram and D30 in 76 patients of 116 patients with both determinations (65.5%). A reduction of 15% or more was evidenced in 18 (11.3%). Of them, 13 (72.2%) had elevated biomarkers in the first 100 days. Of the patients who did not have strain changes, 78.6% had elevated biomarkers. No statistically significant relationship was found between strain reduction and the presence or absence of elevated biomarkers in the first 100 days.
With a median follow-up of 23.3 months (0.89-48.62), 11 (7%) developed clinical cardiotoxicity: hypertension 6, arrhythmia 4, pulmonary embolism 1, sudden death 1. We have strain data of 6/11 patients, and there was no reduction of 15%. Of the 11, 90.9% raised some biomarker during the 100 days. Median development time of cardiotoxicity: 10.3 months (0.03-36.6). 133 had one year follow up so, the incidence of clinical cardiotoxicity at 1 year is 4.5%.
There were no differences in elevated biomarkers in the first 100 days and use of Melphalan (p = 0.096) however, there was a difference with BEAM versus other plans, p = 0.035. The reduction in strain at day 30 was not influenced by Melphalan or BEAM.
Patients with subclinical myocardial damage were older than those without it: mean age: 56.7 +/- 11.2 versus 44.1 +/- 13.1, p = 0.0001. There was no statistically significant difference between patients who had elevated biomarkers in the first 100 days versus those who did not in relation to a history of diabetes, hypertension, dyslipidemia, heart disease or previous use of anthracyclines. Either in patients with a 15% reduction in strain versus those without.
This is one of the first studies worldwide that comprehensively evaluates cardiovascular function during HSCT. Given the small number of observed cardiac complications, greater follow-up of this subpopulation with elements of subclinical cardiotoxicity is required to determine if they are indeed predictive parameters of cardiovascular complications in the future in the transplant setting.
Conclusions:
Subclinical cardiotoxicity is common in transplantation: Pro-BNP is the biomarker that most frequently rises after admission until day 100: 79.1%. Strain reduction of 15% or more occurs in 11,3%. Subclinical myocardial damage parameters were not associated with type of conditioning, previous use of anthracyclines, comorbidities and clinical cardiotoxicity at 1 year. Clinical cardiotoxicity post HSCT is low, 4,5% at 1 year. We must do a longer-term follow-up in order to evaluate whether the combination of pro-BNP associated with strain reduction can be predictive factors of clinical cardiotoxicity.
Oliver: Roche: Other: conference support and fees ; Abbvie: Other: conference support and fees .

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal